
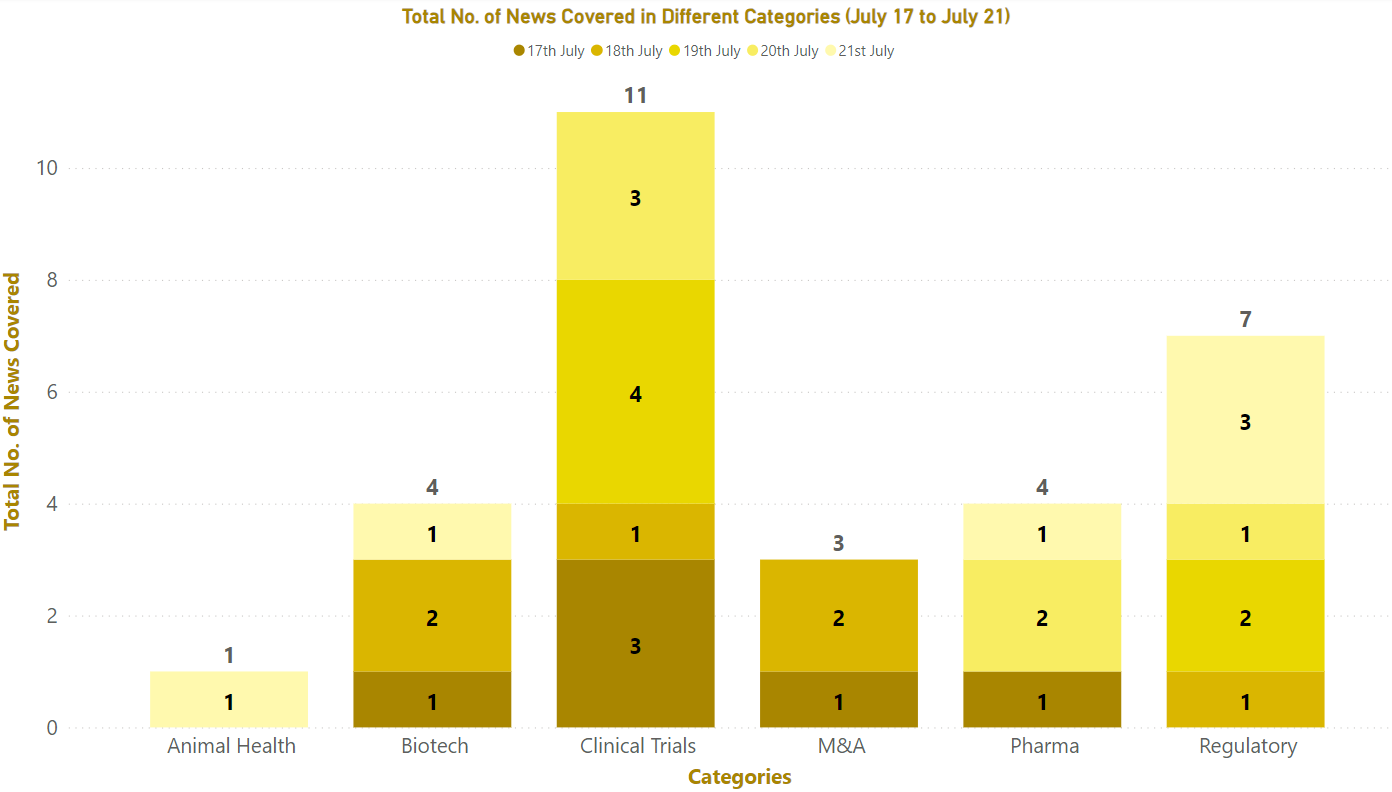
PharmaShots Weekly Snapshots (July 17 – 21, 2023)
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, biotech, pharma, and Animal Health. Check out our full report below:

- Acumen Pharmaceuticals presents the first clinical data from P-I (INTERCEPT-AD) study at AAIC 2023 for ACU193 in AD patients with rapid, dose-related & significant amyloid plaque reduction within higher dose cohorts
Read more: Acumen Pharmaceuticals
- Alphyn Biologics shared positive interim results of AB-101a from the P-IIa Trial for AD treatment with secondary bacterial infection showing itch reduction and improvement in IGA and EASI AD scores
Read more: Alphyn Biologics
- Alnylam highlighted an updated interim result from the P-I study of ALN-APP for AD and Cerebral Amyloid Angiopathy with a rapid reduction in APP production
Read more: Alnylam
- Antengene dosed the first patient in the P-I study (ERASER) of ATG-017 + nivolumab for advanced solid tumors & highlighted preclinical results with synergistic effects in combination with ICIs
Read more: Antengene
- The NMPA accepted Junshi’ sNDA for extensive-stage small-cell lung cancer combination therapy which can significantly improve patients’ PFS and OS
Read more: Junshi Biosciences
- Calidi Biotherapeutics and City of Hope dosed the first patient in the P-I clinical trial of CLD-101 for recurrent high-grade glioma
Read more: Calidi Biotherapeutics and City of Hope
- Faron reported positive results from the P-I/II (BEXMAB) study of bexmarilimab for r/r AML and myelodysplastic syndromes & showed the long duration of the responses
Read more: Faron
- Hansa Biopharma treated the first patient in an investigator-initiated P-II study of Imlifidase, helping more patients suffering from serious autoimmune diseases and conditions
Read more: Hansa Biopharma
- Merck’s Keytruda meets primary endpoint in P-III (KEYNOTE-A18) trial with improvement in PFS over concurrent chemoradiotherapy alone in newly diagnosed high-risk locally advanced cervical cancer patients
Read more: Merck
- Pfizer highlighted P-II study results of Group B Streptococcus (GBS) maternal vaccine candidate GBS6 which generated robust maternal Ab responses against six GBS CPS serotypes & these Abs were efficiently transferred to infants
Read more: Pfizer
- Aurinia highlighted the P-III (AURORA 2) extension study of Lupkynis for LN, published in Arthritis & Rheumatology showing preserved kidney functions with no new or unexpected AEs
Read more: Aurinia

- Eli Lilly to acquire Versanis for ~$1.92B to boost its obesity drug portfolio
Read more: Eli Lilly
- Novartis has acquired DTx Pharma for ~$1B to strengthen Novartis' neuroscience pipeline and expand its capabilities in RNA therapeutics
Read more: Novartis
- Neurogene signed a merger agreement with Neoleukin, focusing on advancing Neurogene’s pipeline of differentiated genetic medicines incl. NGN-401 for Rett syndrome
Read more: Neurogene

- UCB highlighted the US commercial availability of Rystiggo for adult patients with generalized myasthenia gravis along with providing a personalized patient support program ONWARD
Read more: UCB
- Futura Medical announced US licensed deal with Haleon to commercialize innovative topical, gel-based Erectile Dysfunction (ED) treatment MED3000
Read more: Futura Medical
- Biotheus and BioNTech collaborated to discover innovative, potentially transformative therapies for cancer patients
Read more: Biotheus and BioNTech
- Viva Biotech's Portfolio Company Riparian collaborated with Pfizer for novel cardiovascular programs
Read more: Riparian and Pfizer

- Cumulus Oncology and leadXpro collaborated to develop small molecules against novel cancer-focused GPCR targets and use both companies’ expertise
Read more: Cumulus Oncology and leadXpro
- Scribe Therapeutics expands partnership with Sanofi to advance best-in-class genome editing therapies for patients with sickle cell and other genomic disease
Read more: Scribe Therapeutics and Sanofi
- Sangamo Therapeutics entered an option deal with Prevail, a wholly-owned subsidiary of Lilly to evaluate novel capsids for neurological targets
Read more: Sangamo Therapeutics and Prevail
- Recludix Pharma & Sanofi collaborated to advance novel oral STAT6 inhibitors for multiple immunological and inflammatory indications
Read more: Recludix Pharma & Sanofi

- Boehringer Ingelheim’s chewable tablet receives approval in the US for internal and external parasites in dogs which are found to be safe and effective
Read more: Boehringer Ingelheim

- The US FDA’ approved Daiichi Sankyo’s Vanflyta first FLT3 inhibitor for newly diagnosed FLT3-ITD positive AML demonstrating Vanflyta added to CT improved overall survival
Read more: Daiichi Sankyo
- The NMPA has accepted the NDA of SCYNEXIS and Hansoh Pharma’s Ibrexafungerp for adult and post-menarchal pediatric females with vulvovaginal candidiasis
Read more: SCYNEXIS and Hansoh Pharma
- AbbVie’s Tepkinly (epcoritamab) receives EMA’s CHMP positive opinion for r/r DLBCL, based on the P-I/II open-label trial (EPCORE NHL-1)
Read more: AbbVie
- The US FDA accepted NDA for rivoceranib + camrelizumab in unresectable HCC, based on the P-III study (CARES 310) result. The combination therapy is expected to be commercially available shortly
Read more: Elevar Therapeutics
- The NMPA has approved the IND application of Mabwell’s 9MW2921 for the treatment of solid tumor and highlighted that multiple ADC products are expected to enter clinical development in 2023 and 2024
Read more: Mabwell
- Sanofi and AstraZeneca's preventive RSV therapy was approved in the US for infants & is expected to be commercially available in the 2023-2024 RSV season
Read more: Sanofi and AstraZeneca
- The NMPA has granted BTD to HUTCHMED’s fruquintinib + sintilimab for advanced endometrial cancer
Read more: HUTCHMED
Related Post: PharmaShots Weekly Snapshots (July 10 – 14, 2023)
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














